
The crowded environment of the cytosol can accelerate the folding process, since a compact folded protein will occupy less volume than an unfolded protein chain. Macromolecular crowding may be important in chaperone function. Chaperone proteins participate in the folding of over half of all mammalian proteins. Some chaperones can assist in protein degradation, leading proteins to protease systems, such as the ubiquitin-proteasome system in eukaryotes. Ĭhaperones can also work as disaggregases, which interact with aberrant protein assemblies and revert them to monomers.
Other chaperones work as holdases: they bind folding intermediates to prevent their aggregation, for example DnaJ or Hsp33. Although most newly synthesized proteins can fold in absence of chaperones, a minority strictly requires them for the same. Some chaperone systems work as foldases: they support the folding of proteins in an ATP-dependent manner (for example, the GroEL/ GroES or the DnaK/ DnaJ/ GrpE system). The Hsp60 family of protein chaperones are termed chaperonins, and are characterized by a stacked double-ring structure and are found in prokaryotes, in the cytosol of eukaryotes, and in mitochondria. Heat shock protein chaperones are classified based on their observed molecular weights into Hsp60, Hsp70, Hsp90, Hsp104, and small Hsps. Many chaperones are heat shock proteins, that is, proteins expressed in response to elevated temperatures or other cellular stresses. Recent advances in single-molecule analysis have brought insights into structural heterogeneity of chaperones, folding intermediates and affinity of chaperones for unstructured and structured protein chains.įunctions of molecular chaperones Bulk biochemical measurements have informed us on the protein folding efficiency, and prevention of aggregation when chaperones are present during protein folding. Various approaches have been applied to study the structure, dynamics and functioning of chaperones. The specific mode of function of chaperones differs based on their target proteins and location. The majority of molecular chaperones do not convey any steric information for protein folding, and instead assist in protein folding by binding to and stabilizing folding intermediates until the polypeptide chain is fully translated. One major function of molecular chaperones is to prevent the aggregation of misfolded proteins, thus many chaperone proteins are classified as heat shock proteins, as the tendency for protein aggregation is increased by heat stress. The first molecular chaperones discovered were a type of assembly chaperones which assist in the assembly of nucleosomes from folded histones and DNA. Chaperones are also involved in the translocation of proteins for proteolysis. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation.
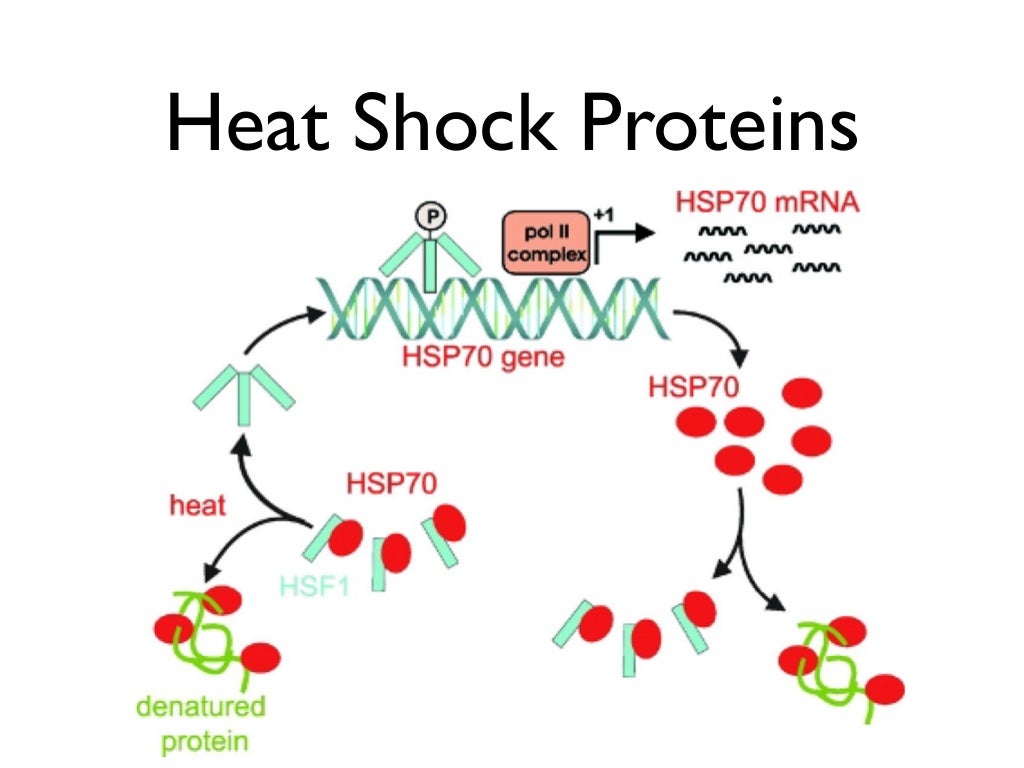
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes.


 0 kommentar(er)
0 kommentar(er)
